The 14th International & 20th National Congress on Quality Improvement in Clinical Laboratories
۱۹ اردیبهشت، ۱۴۰۲
وزیر بهداشت: کلید پیشرفت، حمایت از نخبگان علمی است/ میانگین سرعت رشد علمی 7.44 اما در علوم پزشکی 8.23 است
۱۹ اردیبهشت، ۱۴۰۲Despite progress in human genetics, individuals suffering from rare disorders, such as Duchenne muscular dystrophy (DMD), continue to face a restricted range of treatment options and a shortened lifespan.
The CRISPR-Cas9 technology has enabled scientists to target specific genome regions accurately, enabling precise alterations to disease-causing mutations within human cells.
For clinical purposes, effective and direct delivery of the functional CRISPR components – the Cas9 nuclease and single-guide RNA (sgRNA) – to the patient’s body is imperative.
Research on mice has indicated that adeno-associated viruses can successfully deliver CRISPR-Cas9.
However, there is a concern that prolonged expression from CRISPR-Cas9 DNA vectors may result in adverse effects, such as non-specific cleavage of crucial genes involved in cellular function or unintended mutations in oncogenes that could potentially lead to tumor formation.
In addition, the effectiveness of AAV for delivery may be diminished due to immunological responses. To enable patients to reap the full benefits of recent genomics advancements, clinicians require an alternative delivery system that can transport CRISPR-Cas9 components temporarily.
This system would permit CRISPR-Cas9 to target a specific DNA sequence, make the necessary therapeutic change, and then swiftly degrade to minimize the likelihood of any negative consequences.
Rationale
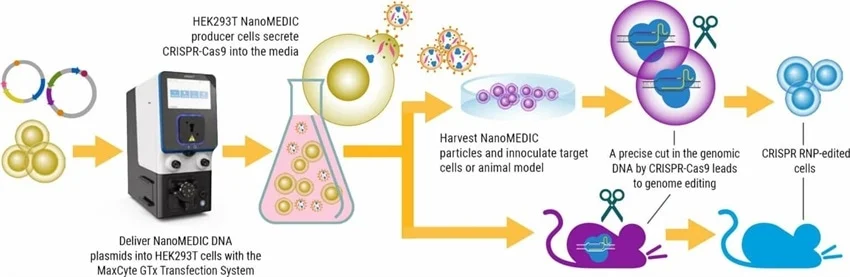
A recent study in Nature Communications introduces a new, temporary delivery system known as NanoMEDIC. This engineered extracellular nanovesicle contains a CRISPR-Cas9 ribonucleoprotein (RNP) complex.
The CRISPR-Cas9 RNP was packaged after removing viral genomic RNA and engineering structural lentiviral components, utilizing a chemical ligand-induced dimerization mechanism. This enabled retention of the delivery properties of lentivirus without the long-term risks associated with viral vector integration.
Despite the feasibility of producing NanoMEDIC at a small scale for basic research, the researchers aimed to develop a scalable approach for its production using a cGMP-compatible Flow Electroporation® system that could be utilized for industrial manufacturing purposes.
Conclusion
The findings of this study demonstrate the potential of a transient CRISPR-Cas9 delivery system not only for gene therapy but also for delivering other cargo, including viral antigens. This technology may have implications for vaccine development, as it could be utilized to elicit a therapeutic immune response.
The manufacturing process developed in this study is devoid of animal components. It employs MaxCyte Flow Electroporation® to facilitate scalable production of NanoMEDIC cells, with the potential to produce billions of cells.
Reference: https://www.news-medical.net/